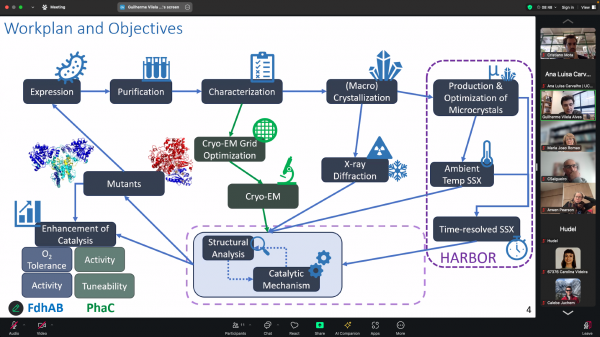
Today, our PhD student Guilherme Vilela-Alves, supervised by Cristiano Mota and Maria João Romão, from XTAL, and Harmut Luecke, from the CryoEM group at NOVA FCT, has publicly presented his PhD Thesis Plan. The whole group is proud of you, Guilherme! Great work and stimulating discussion!