Keep an eye on our blog of HIGHLIGHTS !!!
or follow them on Facebook!
The Macromolecular Crystallography Laboratory is located at the Chemistry Department of the Faculty of Sciences and Technology, Universidade NOVA de Lisboa.
We integrate the Structural Molecular Biology (SMB) Group of UCIBIO, a Research Unit from i4HB, the Associate Laboratory for Health and Bioeconomy.
Main topics of our research |
|
|
|
MOLYBDOENZYMES - MECHANISTIC STUDIES AND IDENTIFICATION OF LIGANDS, AT ATOMIC LEVEL |
ALDEHYDE OXIDASES AND THEIR ROLE IN DRUG METABOLISM |
|
|
|
CELLULOSOMAL PROTEINS. GLYCAN-PROTEIN AND PROTEIN-PROTEIN INTERACTIONS |
NOVEL APPROACHES TO PROTEIN CRYSTALLIZATION USING NANOPARTICLES AND IONIC LIQUIDS |
We use X-ray diffraction data provided by diffractometers and/or synchrotron sources to study protein samples using X-ray Crystallography methods:
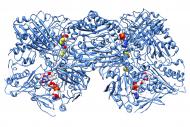
Mechanistic studies and identification of ligands, at atomic level
We have a strong, internationally recognized, expertise in the structural studies of enzymes, like nitrate reductases and aldehyde oxidases from different organisms, and X-ray crystallography methods have helped clarifying the details of several enzymatic mechanisms
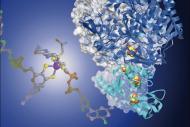
Drug design and ligand discovery
We use several blood plasma proteins to study the recognition (at atomic level) and/or transport of candidate drugs to treat many diseases. Crystallography data has allowed to "view" the protein-drug interactions, at an atomic level
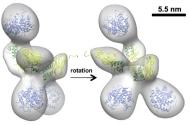
Glycan-protein and protein-protein interactions
We have used X-ray crystallography, associated to other biophysical methods, to study big molecular assemblies that function like nanomachines. X-ray diffraction, cryo-EM and SAXS data have been crucial to characterize protein modular systems, like cellulosomes. Furthermore, glycans are molecules involved in many recognition and communication processes at the cellular and molecular levels and X-ray data, associated to Glycan Microarrays information, allows to "view" molecular interactions with atomic detail, which is helpful to understand how different molecules behave and recognize partners in the cellular environment.
Novel approaches to protein crystallization using nanoparticles and ionic liquids
We use X-ray crystallography methods to assess the effects of innovative agents in crystal growth