Research
Aiming to functionally optimize the respiratory electron-transfer chains, the properties of Geobacter can be explored through genetically engineered strains.
Geobacter species comprise a large number of different multiheme c-type cytochromes involved in the extracellular electron transfer pathways. The functional characterization of multiheme proteins is particularly complex because of the coexistence of several microstates in solution, connecting the fully reduced and oxidized states. NMR spectroscopy has been used to monitor the stepwise oxidation of each individual heme and thus to obtain information on each microstate. For the structural study of these proteins, a cost-effective isotopic labeling of the protein polypeptide chains was combined with the comparative analysis of 1H-13C HSQC (heteronuclear single-quantum correlation) NMR spectra obtained for labeled and unlabeled samples.
These new methodological approaches allowed us to study G. sulfurreducens and G. metallireducens heme proteins functionally and structurally, revealing functional mechanisms and key residues involved in their electron transfer capabilities.
Such advances can now be applied to the design of engineered heme proteins to improve the bioremediation and electricity-harvesting skills of Geobacter.
The targets that are currently under research in our laboratory are the following:
Triheme cytochromes
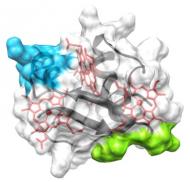 |
The members of this family are periplasmic cytochromes that have been shown to be directly involved in metabolic pathways that lead to removal of Fe(III), U(VI) and Cr(VI). This family is composed by five triheme cytochromes in both G. sulfurreducens and G. metallireducens and is thought to be essential to bridge the electron transfer from the cytoplasmic donors to the extracellular acceptors. Their study aims to understand the structure-function mechanisms within each individual member but also how they interact with physiological partners in the electron transfer chain of Gs. |
"Nanowire" cytochromes c
 |
Gs' genome contains three ORFs encoding two proteins with 12 heme binding motifs and one with the unprecedented number of 27 heme binding motifs. These proteins can be divided into three-heme units that are homologous to each other within the same protein. These proteins represent a new class of cytochromes c that is characterized by multidomain composition consisting of highly homologous modules and by a mixed type of heme coordination. |
Outer membrane cytochromes
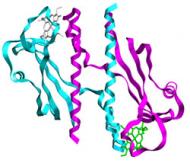
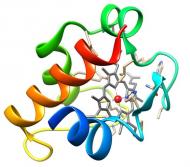 |
The members of this family are quite diverse. Genetic experiments have shown their involvement in the electron transfer pathways that lead to metal reduction in Gs. Therefore they are proposed to act as terminal reductases on these pathways being crucial for metal bioremediation and energy production. |
Signal transduction heme proteins
 |
Gs has an unusually large number of signal transduction proteins. Ten periplasmic sensor domains were identified in Gs' genome containing at least one heme c-binding motif. They are part of proteins annotated as two-component signal transduction or chemotaxis proteins. |
Monoheme periplasmic cytochromes
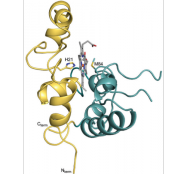 |
Monoheme cytochromes have been associated to different metabolic pathways in Gs, as distinct as extracellular electron transfer to specific terminal acceptors or as electron transfer from electrodes in microbial electrosynthesis processes. |
Membrane associated cytochromes
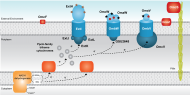 |
Porin-cytochrome c protein complexes were recently identified in Gs and proposed to mediate electron transfer across the outer membrane. These complexes are formed by and integral outer membrane protein that anchors a periplasmic multiheme cytochrome to an outer surface cytochrome. |