Context & Impact

This work is supported by national funds through FCT – Fundação para a Ciência e a Tecnologia, I.P., under the project reference 2022.06104.PTDC.
Mucin-type O-glycosylation is a post-translational modification of proteins that plays a crucial role in the molecular and cell biology processes occurring in cancer. The aberrant O-glyco-phenotype of cancer cells is known to harbour a ‘glyco-code’ (i.e. specific structures of glycans), that alters the function of their carrier proteins or is recognised by receptors of the immune system to promote signalling events in cancer development and progression. This knowledge has prompted research to explore mucin-type O-glycan structures for diagnosis and for development of cancer vaccines and innovative immunotherapies.
Despite major advances made by members of the team and by others in the world-leading laboratories in using O-glycoproteomic and functional O-glycomic approaches to unravel the complexity of O-glycosylation and recognition of O-glycans in disease, there is a gap in knowledge of the molecular structures and biological activities of cancer-associated O-glycoproteins/glycopeptides. Therefore, new tools are required in O-glycoproteomics and functional O-glycomics to enable the identification and characterization of carrier proteins and O-glycan structures, and advance studies of their role in carcinogenesis and their disclosure as putative biomarkers with potential clinical applications.
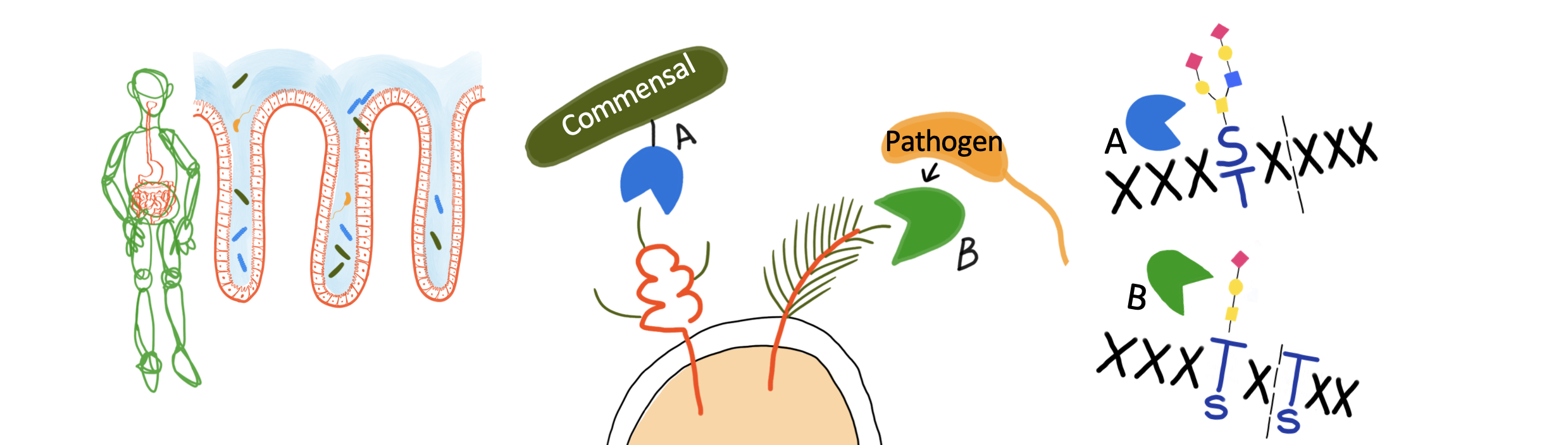
Microorganisms (commensal and pathogenic) of the human microbiome have adapted to the gastrointestinal environment by developing enzymatic systems that specifically target mucin O-glycoproteins at the host epithelial mucus layer, both as nutrients or as adhesion sites to initiate infection. The biochemical characterization of these systems is unravelling mechanisms of commensal and pathogen interactions with host. New enzymes with specificities to mucin-type O-glycan structures are being identified. These studies have revealed metallopeptidases selective for O-glycans and demonstrated that these are effective tools for analysis of mucin-type O-glycoproteins. This evidence highlights the importance to discover novel selective enzymes from bacteria that are highly specialized in mucin degradation.
In GlycOSELECT, we aim to develop a ‘toolbox’ of O-Glycan-Selective metallopeptidases identified in the genome of mucin-degrading bacteria to advance biological approaches for molecular O-glycoproteomic and O-glycomic studies in cancer. To this end, GlycOSELECT will focus on a molecular and structural comparative analysis of modular metallopeptidases (with catalytic and binding domains) identified in commensal and pathogenic bacteria. The hypothesis is that the enzymes of pathogens exhibit O-glycan and peptide-backbone selectivity different from those of commensals to target mucin-type O-glycoproteins, enabling a functionally diverse panel of proteins to build the toolbox.
 |
 |
|
 |
|
 |